Background: High-dose chemotherapy followed by autologous hematopoietic cell transplantation (auto HCT) is considered to be the standard of care treatment for transplant-eligible patients with multiple myeloma (MM). The most commonly used conditioning regimen in this setting is high-dose melphalan by intravenous administration. Conventional melphalan formulations, when administered at high doses, can put patients at risk of potential propylene glycol-associated toxicities. Melphalan for injection (Evomela) is propylene glycol free (PGF), can be dissolved directly using saline, and as a PGF reformulation of Alkeran, incorporating Captisol brand of beta-cyclodextrin sulfobutyl ethers sodium salts, overcomes previous formulation limitations. In 2016 Evomela was the first product approved by the US FDA for high-dose conditioning treatment prior to HCT in MM patients and it is also indicated for the palliative treatment of patients with MM for whom oral therapy is not appropriate.
Reconstituted Evomela solution can be stored in the vial for up to 1 hour at room temperature or up to 24 hours at 2-8 °C with no significant degradation. After storage in the vial, it remains stable for an additional 3 to 29 hours after, preparation of admixture solution in infusion bags at concentrations of 0.25 to 5.0 mg/mL, respectively. As well, Evomela solution in saline, at concentration of 5.0 mg/mL melphalan, was bacteriostatic through 72 hours when stored at 2-8 °C. This stability allows for less frequent handling by pharmacy and nursing staff, resulting in a concomitant decrease in exposure risks, increased convenience and administration flexibility, suggestive of an improved ease of handling and administration, when compared to Alkeran. Further, Evomela may actually be less toxic due to the absence of propylene glycol.
Although increased melphalan doses have previously demonstrated signals of improved response, the most commonly used dose of melphalan is 200 mg/m2, primarily due to concerns of toxicity. Emerging data regarding higher stability and potentially less toxicity of PGF melphalan (Evomela) supports dose escalation evaluation in order to improve the outcomes.
Previous trial data have shown that continuous infusion or frequent fractionated-dose delivery increases the antitumour activity of several drugs. Due to the instability of currently available Alkeran at the room temperature, infusional studies have not been feasible. This limitation is overcome by Evomela, being that the compound is stable for several hours at the room temperature, thus allowing evaluation of infusional schedules in addition to the traditional 30-60 minute bolus doses.
Here, we describe a trial designed to assess whether the above noted characteristics of Evomela allow for the escalation of dose and prolongation of infusion time, in order to increase the efficacy of melphalan in patients undergoing auto-HCT.
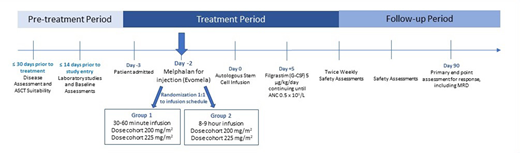
Study Design/Methods: This is a two-stage phase I-II trial to optimize the dose and schedule of Evomela given as a single agent preparative regimen for auto-HCT. Up to 60 participants may be included if they are 18-70 years of age, with non-relapsed MM, have a Karnofsky performance score ≥70%, who have received at least two cycles of initial systemic therapy, and are within 2 to 12 months of the first dose.
Patients will be randomized 1:1 to two different infusion schedules (30-60 minute infusion or 8-9 hour infusion) using Evomela (2mg/ml) at two dose cohorts (200mg/ml2 or 225mg/m2). Because 2 mg/mL Evomela is stable for 10 hours, patients receiving the 8-9 hour infusion will receive the total dose in one single infusion bag. The primary objectives are to determine the optimal dose and schedule of Evomela before auto-HCT for MM and collect pharmacokinetic data and compare the exposure-response evaluations between the two infusion schedules. Secondary outcomes will include incidence of treatment-related mortality, rate of minimal residual disease negative complete response at 90 days post auto-HCT, progression-free survival and overall survival after auto-HCT in newly diagnosed myeloma patients treated on different schedules and doses of Evomela. The active study follow-up period will be up to one-year post auto-HCT.
Bashir:Acrotech: Research Funding; StemLine: Research Funding; Takeda: Other: Advisory Board, Research Funding; Celgene: Research Funding; KITE: Other: Advisory Board; Amgen: Other: Advisory Board; Purdue: Other: Advisory Board. Qazilbash:Amgen: Research Funding; Bioclinica: Consultancy; Angiocrine: Research Funding; Bioline: Research Funding; Janssen: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal